Sampling from saliva and oral cavity .
The OMNIgene® ORAL product line includes several kits for collecting samples of the oral microbiome.
The kits are especially suited for self-testing. DNA and RNA are stabilized so well within the tube that no cold chain is required during storage and transportation.
Hard Facts .
- Easy self collecting
- High-quality DNA/RNA suited for all common downstream applications
- Best possible representation of the microbiome
- DNA and RNA stability up to 4 weeks

Applications .
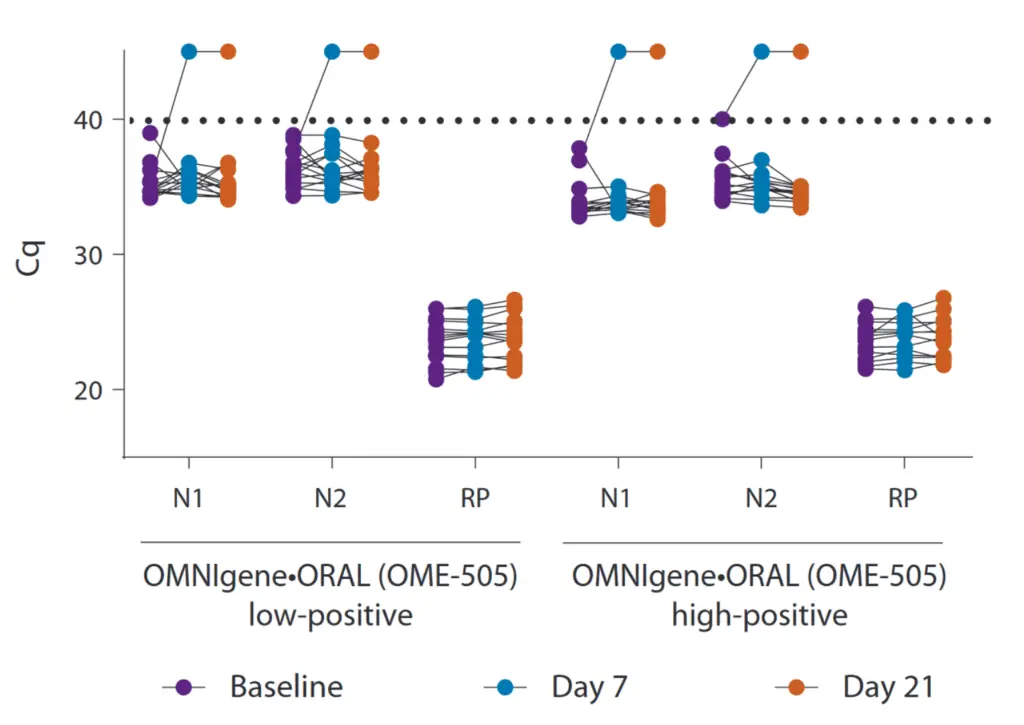
Cq values for SARS-CoV-2 RT-qPCR targets N1, N2, and RP at day 0 (purple), day 7 (blue), and day 21 (orange) in low-positive (500 cp/ml, N = 15) and high-positive (2,500 cp/ml, N = 14) enriched saliva samples. The dotted line indicates the detection limit. If no amplification was detected, the cq was set to 45 (maximum cq) for visualization. Statistical significance was determined using a two-way ANOVA with Sidak’s test for multiple comparisons.
Colameco, S., Brown, A., & Crawford Parks, T. (2020). Ambient temperature stabilization of SARS-CoV-2 viral RNA in OMNIgene®-ORAL (OME-505) collected saliva samples. DNA Genotek https://www.dnagenotek.com/ROW/pdf/PD-WP-00069.pdf
OMNIgene ORAL OME-505 is ideal for the collection and stabilization of saliva samples. Even after 21 days of storage at room temperature, still the smallest amounts of viral RNA can be detected.
Colameco, S., Brown, A., & Crawford Parks, T. (2020). Ambient temperature stabilization of SARS-CoV-2 viral RNA in OMNIgene®-ORAL (OME-505) collected saliva samples. DNA Genotek https://www.dnagenotek.com/ROW/pdf/PD-WP-00069.pdf
OMNIgene ORAL products offer a painless, non-invasive alternative to blood collection for the detection of circulating Plasmodium falciparum or P. vivax.
Mfuh, K. O., Tassi Yunga, S., Esemu, L. F., Bekindaka, O. N., Yonga, J., Djontu, J. C., Mbakop, C. D., Taylor, D. W., Nerurkar, V. R., & Leke, R. G. F. (2017). Detection of Plasmodium falciparum DNA in saliva samples stored at room temperature: potential for a non-invasive saliva-based diagnostic test for malaria. Malaria Journal, 16(1), 434. https://doi.org/10.1186/s12936-017-2084-5
Okumu, W., Okeyo, W. A., Munde, E. O., Raballah, E., Anyona, S. B., & Ouma, C. (2015). Evaluation of saliva-derived Plasmodium falciparum DNA using the OMNIgene®-ORAL kit in detection of malaria. DNA Genotek https://www.dnagenotek.com/ROW/pdf/PD-WP-00035.pdf
Visanuvimol, D. (2014). Saliva as a proven, non-invasive sample type for molecular malaria testing and surveillance using OMNIgene®-ORAL at ambient temperatures. DNA Genotek https://www.dnagenotek.com/ROW/pdf/PD-WP-00041.pdf
Details .
Product overview .
| Attributes | OMR-110 | OMR-120 | OM-501 | OME-505 | ORE-100 |
|---|---|---|---|---|---|
| Collection site | Gum and plaque | Tongue | Saliva | Saliva | Oral cavity |
| High quality nucleic acids | DNA and RNA | DNA and RNA | DNA | DNA and RNA | RNA |
| Microbiome profile stability at room temperature | 4 weeks | 4 weeks | 1 year | 3 weeks | 1 year |
| Eliminate cold chain shipping | ✔ | ✔ | ✔ | ✔ | ✔ |
| Standardized format for high throughput processing | ✔ | ✔ | ✔ | ✔ | ✔ |
| CE-IVD certified | ✔ | ✔ | |||
| Number of extractions per kit | 2 | 2 | 8 | 8 | |
| Suitable for NGS downstream applications | ✔ | ✔ | ✔ | ✔ | ✔ |
| Swab-based collection | ✔ | ✔ | ✔ |

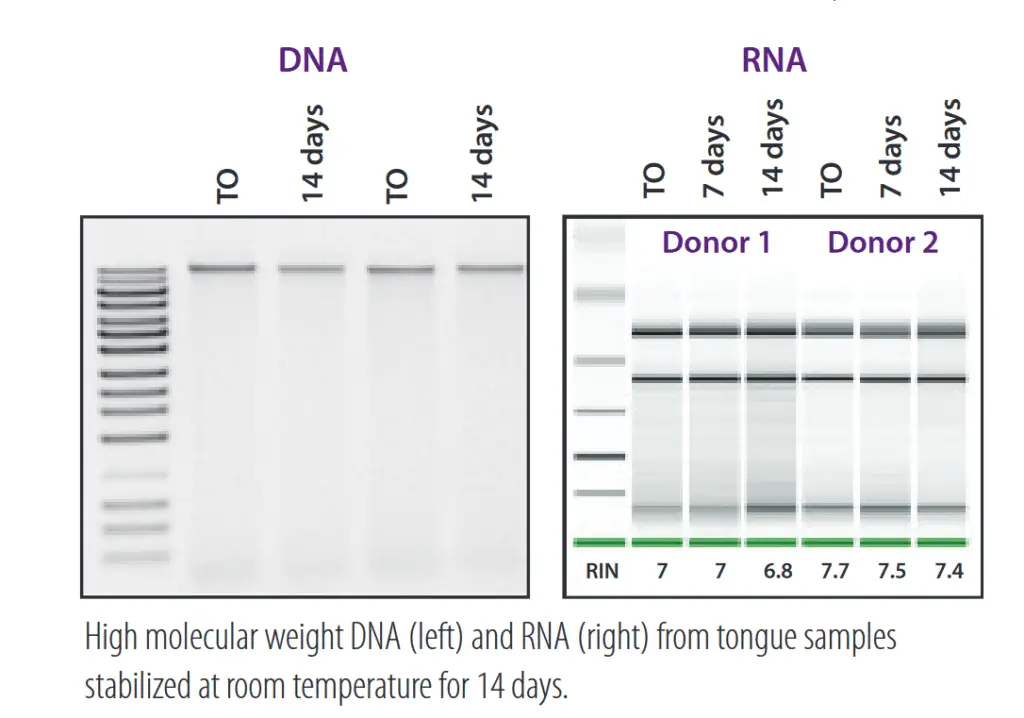
Outstanding stability .
DNA and RNA in the sample are stable for a long time at room temperature in OMR-110, OMR-120, OM-501 and OME-505 kits. This simplifies the transport chain and prevents microbiome profile distortion caused by microbial growth or nucleic acid degradation.
From: DNA Genotek. (n.d.). Optimized solutions for sample collection, nucleic acid stabilization and microbial profile snapshot. https://www.dnagenotek.com/ROW/pdf/PD-BR-00205.pdf
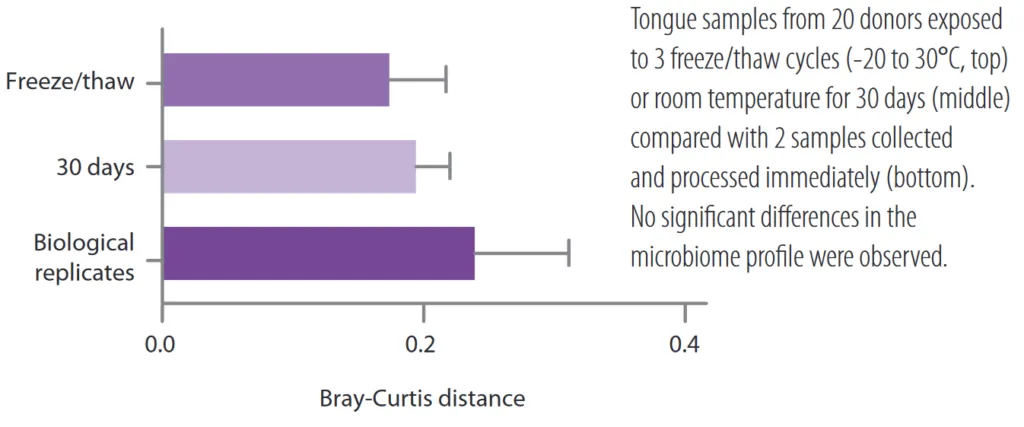
Realistic snapshot of the microbiome .
Samples obtained with OMNIgene kits are of excellent quality. The high integrity of the stabilized DNA allows a very realistic representation of the microbiome profile.
From: DNA Genotek. (n.d.). OMNIgene Devices. https://www.dnagenotek.com/ROW/pdf/PD-BR-00202.pdf
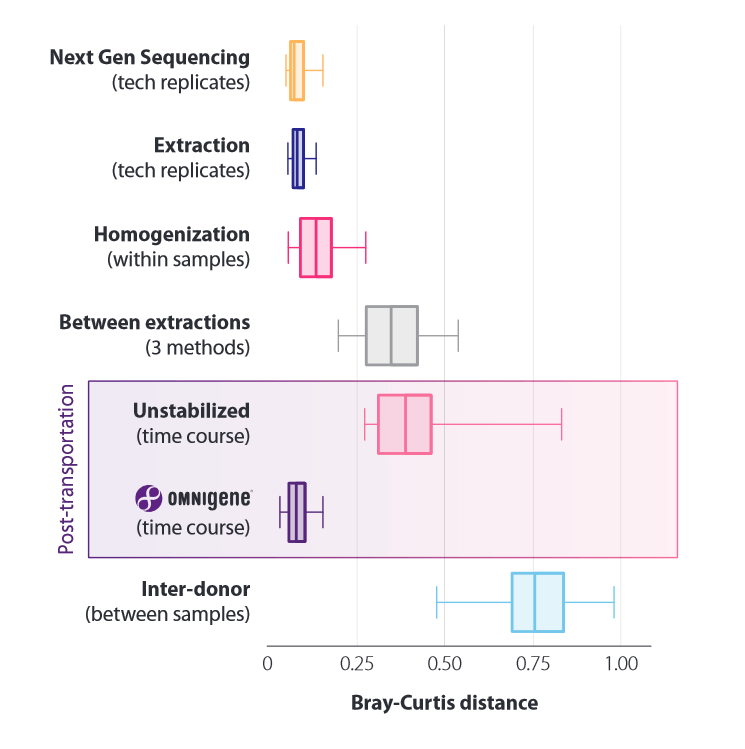
Quantifying the effects of pre-analytical factors: microbiome profile differences between technical replicates during sequencing, extraction, sample homogenization, of 3 different kits compared. Analysis was performed with respect to transport (temperature fluctuations between -8°C and +23°C, unstabilized and OMNIgenes) and donor to donor (control).

ORE-100 .
Every expression analysis starts with the collection of RNA samples. ORAcollect® RNA (ORE-100) is an all-in-one system for the collection, stabilization and transport of RNA from saliva for in vitro diagnostics.
Advantages .
- Stabilizes samples during collection and ensures that DNA and RNA are unchanged at the time of processing
- Stabilizes human, viral and bacterial RNA from saliva at room temperature
- Reduces transportation and storage costs and complexity with up to 60 days stability of RNA at room temperature
- Easy self-collection of high-quality DNA and RNA from oral samples
- No refrigerated transports necessary
- Compatible with downstream applications (e.g. RT-qPCR, microarray)
- Format suitable for high throughput processing
More information .
-
Manufacturer websiteManufacturer website
-
Instruction manual (engl.)Instruction manual (engl.)

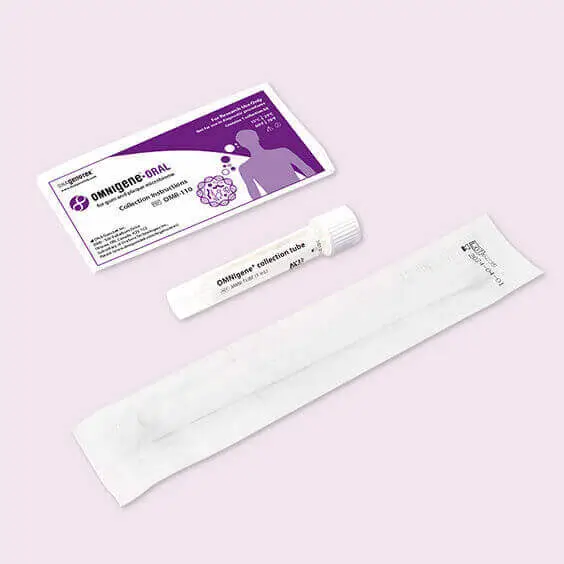
OMR-110 .
OMNIgene-ORAL OMR-110 is a system for easy sampling for microbiome profile analyses of gums and plaque. Minimized bias and reduced data noise ensure the most realistic microbiota profile possible.
Advantages .
- Easy self-sampling by patients or study participants
- Effective stabilization of nucleic acids
- High quality and integrity of recovered DNA and RNA for up to 30 days without refrigeration
- Drastic reduction in shipping and storage costs, as no cooling is required
- Compatible with all common molecular downstream applications
More information .
-
Manufacturer websiteManufacturer website
-
Instruction manual (engl.)Instruction manual (engl.)

OMR-120 .
OMNIgene-ORAL sampling vessel for easy self-collection and stabilization of DNA and RNA for tongue microbiome profile analysis.

Advantages .
- Easy self-collection of high-quality DNA and RNA from tongue swabs
- Provides a snapshot of the microbial profile at the time of collection
- Minimization of distortions caused by microbial growth and nucleic acid degradation.
- Minimize noise in your data analysis with a reliable microbiota profile
- Compatible with molecular downstream applications
- Optimal sample acquisition and release
- Preservation of DNA and RNA integrity under typical ambient temperature fluctuations (e.g. -20°C to 30°C) for up to 30 days.
More information .
-
Manufacturer websiteManufacturer website
-
Instruction manual (engl.)Instruction manual (engl.)

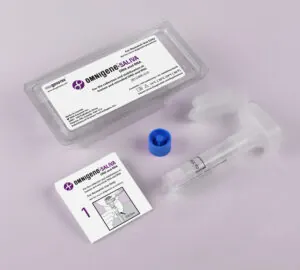
OMR-610 .
The OMNIgene-SALIVA DNA and RNA Kit is validated for self-collection of saliva and total nucleic acid stabilization. Single sample analysis with a true multi-omics collection kit sets a new standard for home collection of total nucleic acids from saliva.
Advantages .
- Collection of human and microbial DNA and RNA in one kit
- DNA and RNA is stable at room temperature for up to 21 days
- Equivalent to blood for DNA analysis
- Painless and reliable self-sampling
- Optimized for both manual sample processing and automated workflows
- Integrated 1D barcode for efficient workflow
- Compatible with downstream applications, including PCR/RT-PCR
More information .
-
Manufacturer websiteManufacturer website
-
Instruction manual (engl.)Instruction manual (engl.)
-
Data SheetData Sheet

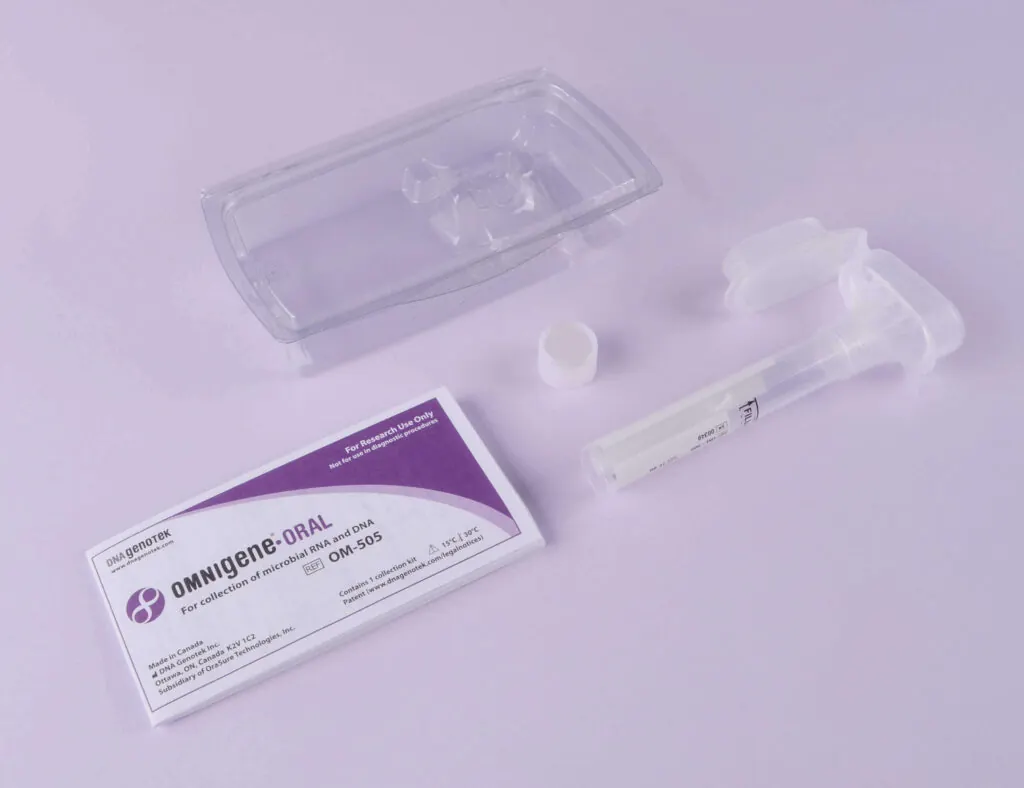
OME-505 .
OMNIgene ORAL OME-505 is perfect for the collection and stabilization of microbial nucleic acids. The kit provides excellent sample integrity and stabilization. This is essential to exclude bias when analyzing the microbiome using microarrays or next generation sequencing.
Advantages .
- CE/IVD certified
- Easy self-collection of high-quality DNA and RNA from oral samples
- Stabilizes samples from collection to analysis of DNA and RNA - no refrigerated transport necessary
- Snapshot of the microbial profile at the time of sampling.
- Allows simultaneous detection of DNA and RNA of bacteria and viruses from a single sample
- Reduces transport and storage costs and process complexity due to 3 weeks of room temperature stability of DNA and RNA
- Allows identification of live or metabolically active bacteria at the time of collection by RNA expression profiling
- Tube format is suitable for high-throughput analysis, increases efficiency and minimizes sample handling errors
- Compatible with downstream applications (e.g. RT-qPCR, microarray)
| Application | Target | Reference |
|---|---|---|
| Virus | DNA | EBV [1], HHV-8 [2,3], CMV [4] |
| RNA | HCV [5], HIV [5,6] | |
| Bacteria | DNA | HOMIM [7] |
| RNA | E. coli [9] | |
| Extraction systems | Abbott m2000 [5,6], Qiagen MinElute [8] | |
| Downstream applications | qPCR [1,2,3], Microarray [7] |
[2] High quality microbial DNA collected with OMNIgene•ORAL enables detection of HHV-8 in saliva. DNA Genotek. MK-00018.
[3] Evaluation of a new commercial kit for detection of human herpesvirus 8 (HHV-8) in saliva. http://www.dnagenotek.com/ROW/pdf/GriffithUniversity.pdf
[4] Genotyping of cytomegalovirus from toddlers’ saliva samples collected with OMNIgene•ORAL. DNA Genotek. MK-00050.
[5] Compatibility of saliva collected using OMNIgene•ORAL (OM-505) with the Abbott m2000rt RealTime System for the detection of viral RNA. DNA Genotek. PD-WP-00025.
[6] OMNIgene•ORAL (OM-505) stabilizes RNA from human viruses at room temperature for several weeks. DNA Genotek. PD-WP-00024.
[7] OMNIgene•ORAL stabilizes microbial DNA profiles in oral fluid samples, enables more precise characterization of oral flora. DNA Genotek. MK-00090.
[8] OM-505 Microbial DNA and RNA purification protocol using QIAGEN® QIAamp® MinElute Virus Spin Kit. DNA Genotek. PD-PR-00214.
[9] Internal validation using OM-505 extraction8 in house RT-qPCR.
More information .
-
Manufacturer websiteManufacturer website
-
Instruction manual (engl.)Instruction manual (engl.)

OM-501 .
The OMNIgene ORAL OM-501 kit enables the collection of high-quality samples for the molecular detection of microbial DNA. Samples are preserved well enough to avoid bias until analysis by microarrays or next generation sequencing.
Advantages .
- Improved patient compliance through painless, non-invasive specimen collection
- Increases specimen handling efficiency and reduces handling errors
- Ideal for on-site collection, transport at room temperature and long-term storage
- The sample remains stable for one year at room temperature, which reduces the cost and effort of transport and storage
- Format suitable for high throughput automated processing
| Venous blood | Mouthwash | Buccal swabs | OMNIgene•ORAL (OM-501) | |
|---|---|---|---|---|
| Non-invasive collextion | ✘ | ✘ | ✔ | ✔ |
| Standardized format for high-throughput processing | ✔ | ✘ | ✘ | ✔ |
| Specimen stability at room temperature | Days | Weeks | Days | 1 year |
| Blood collection | Oral collection |
More information .
-
Manufacturer websiteManufacturer website
-
Instruction manual (engl.)Instruction manual (engl.)