MagSi-STA - Bead meets Streptavidin .
Magnetic particles are ideal as a solid support phase for immunoassays. They enable simplified workflows and automated processes.
Our superparamagnetic MagSi-STA silica beads are coated with a modified streptavidin. This makes them ideal for purification or capture of biotinylated molecules.
Hard Facts .
- Streptavidin-coupled magnetic beads.
- High binding capacity of up to 6800 pmol biotin/mg beads
- Simple handling
- Short and fast protocol
- Low non-specific interactions
- Different bead sizes available

Applications .
MagSi-STA beads are perfect for capturing biotinylated molecules. Molecules bound to the beads can be removed or purified from the sample using simple protocols.
The MagSi-STA beads are ideally suited as solid support phase of immunoassays. In “sandwich assays”, e.g. antigens or biotinylated antibodies can be detected very specifically.
Schmalenberg, M., Beaudoin, C., Bulst, L., Steubl, D., & Luppa, P. B. (2015). Magnetic bead fluorescent immunoassay for the rapid detection of the novel inflammation marker YKL40 at the point-of-care. Journal of Immunological Methods, 427, 36-41. https://doi.org/10.1016/j.jim.2015.09.004
When gene expression profiling using RNA-Seq, interfering rRNA must be removed. For this “depletion”, one uses sequence-specific biotinylated oligonucleotides that bind to strepdavidin beads such as MagSi-STA 600 BI. These can then be easily removed from the reaction mixture by magnet.
Antigens can also be elegantly and very specifically captured with biotinylated antibodies. The resulting antigen-antibody complexes bind to streptavidin-coupled beads and can then be easily and rapidly purified or concentrated.
The fishing of specific cells for further cell line analysis is greatly simplified by Magnetic Beads. This involves mixing cells with cell line-specific biotinylated antibodies and then fishing them with streptavidin-coupled MagSi-STA.
Wirth, F., Huck, K., Lubosch, A., Zoeller, C., Ghura, H., Porubsky, S., & Nakchbandi, I. A. (2021). Cdc42 in osterix-expressing cells alters osteoblast behavior and myeloid lineage commitment. Bone, 153, 116150. https://doi.org/10.1016/j.bone.2021.116150
Details .
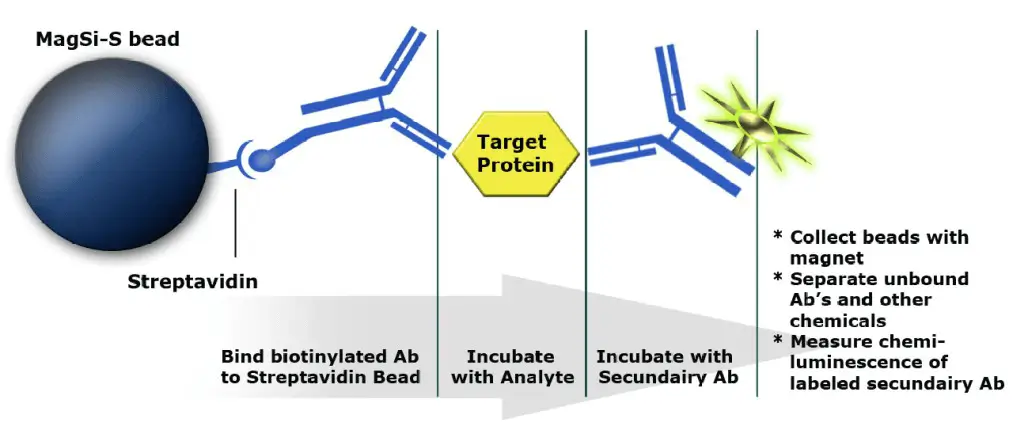
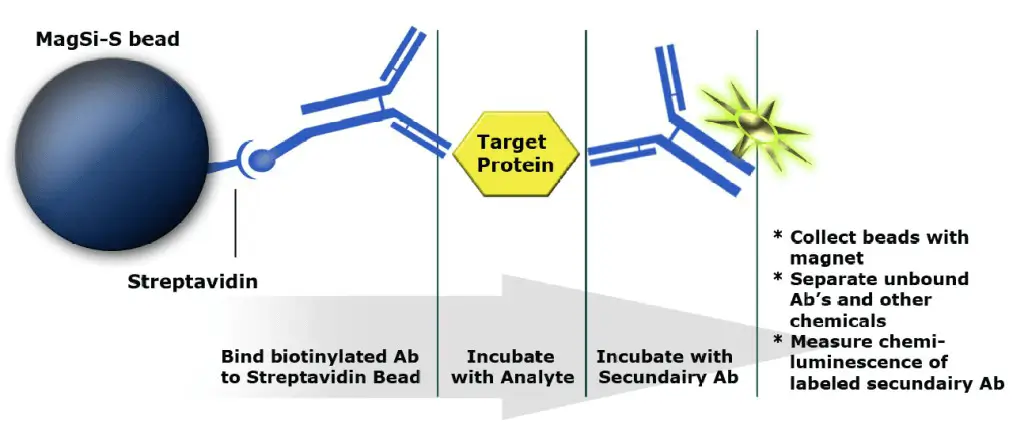
Magnetic beads as solid phase in immunoassays .
Sandwich assay for the detection of antigens:
- Biotinylated antibody binds to bead (solid phase)
- Target protein / antigen binds to antibody and is thus fished out of solution
- Secondary antibody with reporter (HRP) binds to target/antigen. This complex is detected via the reporter’s chemiluminescent signal.
MagSi-STA .
MagSi-STA beads are available in two types with different streptavidin coupling chemistry.
Carboxyl (C) coupled beads – with additional spacer – are suitable for applications that require a more hydrophilic surface.
Tosyl (T) coupled beads are optimized for applications that require a more hydrophobic surface.
| Product | Coupling chemistry | Concentration | Size | Binding capacity (pmol biotin/mg beads) |
|---|---|---|---|---|
| MagSi-STA 600 | Carboxyl | 10 mg/ml | 600 nm | 3500-5000 |
| MagSi-STA 600 BI | Carboxyl | 10 mg/ml | 600 nm | 6000-6800 |
| MagSi-STA 1.0 | Carboxyl | 10 mg/ml | 1,0 µm | 3500-5000 |
| MagSi-STA 1.0 L | Carboxyl | 10 mg/ml | 1,0 µm | 1200-2000 |
| MagSi-STA 3.0 L | Carboxyl | 10 mg/ml | 3,0 µm | 700-1200 |
| MagSi-STA 1.0 TL | Tosyl | 10 mg/ml | 1,0 µm | 1200-2000 |
| MagSi-STA 1.0 TS | Tosyl | 10 mg/ml | 1,0 µm | 3500-5000 |
| MagSi-STA 3.0 TL | Tosyl | 10 mg/ml | 3,0 µm | 500-900 |
Capture and depletion of biotinylated proteins .
Capturing biotinylated molecules using MagSi-STA Magnetic Beads is super simple. The protocol consists of only three steps: bind, wash, elute.

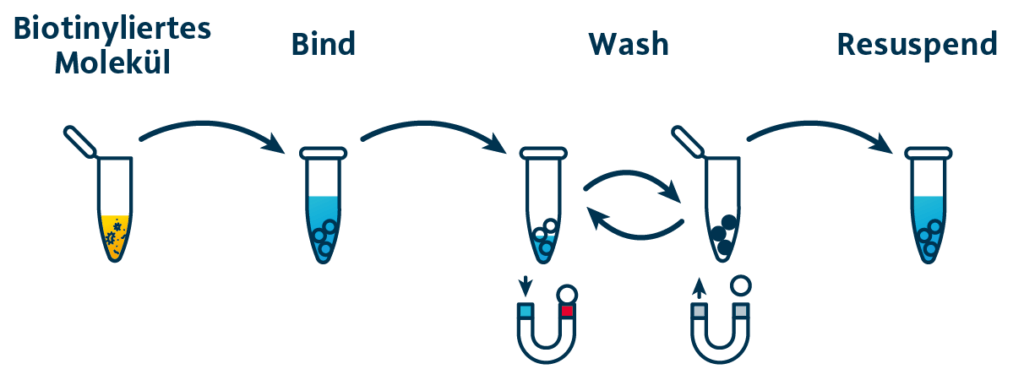
The MagSi-STA beads with surface-bound streptavidin are added to the solution containing the biotinylated analyte. After a short incubation, the beads are collected by magnets, washed several times and resuspended.
MagSi-STA Beads with Carboxyl Coupling .
MagSi-STA beads are available in two different types – depending on the streptavidin coupling chemistry. Carboxyl-coupled beads – with additional spacer- are suitable for applications that require a more hydrophilic surface.
| Item number | Volume | Product | Concentration | Size | Binding capacity (pmol biotin/mg beads) |
|---|---|---|---|---|---|
| MD16001 | 2 ml | MagSi-STA 600 | 10 mg/ml | 600 nm | 3500-5000 |
| MD18001 | 10 ml | ||||
| MD19001 | 100 ml | ||||
| MD21001 | 2 ml | MagSi-STA 600 BI | 10 mg/ml | 600 nm | 6000-6800 |
| MD31001 | 10 ml | ||||
| MD41001 | 100 ml | ||||
| MD01001 | 2 ml | MagSi-STA 1.0 | 10 mg/ml | 1.0 µm | 3500-5000 |
| MD03001 | 10 ml | ||||
| MD04001 | 100 ml | ||||
| MD06001 | 2 ml | MagSi-STA 1.0 L | 10 mg/ml | 1.0 µm | 1200-2000 |
| MD07001 | 10 ml | ||||
| MD08001 | 100 ml | ||||
| MD33001 | 2 ml | MagSi-STA 3.0 L | 10 mg/ml | 3.0 µm | 700-1200 |
| MD34001 | 10 ml | ||||
| MD35001 | 100 ml |
MagSi-STA Beads with tosyl coupling .
MagSi-STA tosyl beads are optimal for applications that require a hydrophobic surface.
| Item number | Volume | Product | Concentration | Size | Binding capacity (pmol biotin/mg beads) |
|---|---|---|---|---|---|
| MD25001 | 2 ml | MagSi-STA 1.0 TL | 10 mg/ml | 1.0 µm | 1200-2000 |
| MD26001 | 10 ml | ||||
| MD27001 | 100 ml | ||||
| MD29001 | 2 ml | MagSi-STA 1.0 TS | 10 mg/ml | 1.0 µm | 3500-5000 |
| MD30001 | 10 ml | ||||
| MD31001 | 100 ml | ||||
| MD37001 | 2 ml | MagSi-STA 3.0 TL | 10 mg/ml | 3.0 µm | 500-900 |
| MD38001 | 10 ml | ||||
| MD39001 | 100 ml |
MagSi-STA Beads .
Available in 600 nm, 1.0 µm and 3.0 µm sizes with carboxyl or tosyl surface.
Downloads .
Martin, J. G., Gupta, M., Xu, Y., Akella, S., Liu, J., Dordick, J. S., & Linhardt, R. J. (2009). Toward an artificial Golgi: redesigning the biological activities of heparan sulfate on a digital microfluidic chip. Journal of the American Chemical Society, 131(31), 11041-11048. https://doi.org/10.1021/ja903038d
Schmalenberg, M., Beaudoin, C., Bulst, L., Steubl, D., & Luppa, P. B. (2015). Magnetic bead fluorescent immunoassay for the rapid detection of the novel inflammation marker YKL40 at the point-of-care. Journal of Immunological Methods, 427, 36-41. https://doi.org/10.1016/j.jim.2015.09.004
Chicher, J., Simonetti, A., Kuhn, L., Schaeffer, L., Hammann, P., Eriani, G., & Martin, F. (2015). Purification of mRNA-programmed translation initiation complexes suitable for mass spectrometry analysis. Proteomics, 15(14), 2417-2425. https://doi.org/10.1002/pmic.201400628
Martin, F., Ménétret, J.-F., Simonetti, A., Myasnikov, A. G., Vicens, Q., Prongidi-Fix, L., Natchiar, S. K., Klaholz, B. P., & Eriani, G. (2016). Ribosomal 18S rRNA base pairs with mRNA during eukaryotic translation initiation. Nature Communications, 7, 12622. https://doi.org/10.1038/ncomms12622
Shah, T., Qin, S., Vashi, M., Predescu, D. N., Jeganathan, N., Bardita, C., Ganesh, B., diBartolo, S., Fogg, L. F., Balk, R. A., & Predescu, S. A. (2018). Alk5/Runx1 signaling mediated by extracellular vesicles promotes vascular repair in acute respiratory distress syndrome. Clinical and Translational Medicine, 7(1), 19. https://doi.org/10.1186/s40169-018-0197-2
Wirth, F., Huck, K., Lubosch, A., Zoeller, C., Ghura, H., Porubsky, S., & Nakchbandi, I. A. (2021). Cdc42 in osterix-expressing cells alters osteoblast behavior and myeloid lineage commitment. Bone, 153, 116150. https://doi.org/10.1016/j.bone.2021.116150