
DNA purification with magnetic beads .
Magnetic beads are an ideal tool for fast and easy purification of DNA and RNA from plants and animal or human sample material.
In genetic analyses – especially in diagnostics or forensics – a high purity of DNA is necessary. Our Magnetic Beads excellently meet this requirement and reliably remove proteins, lipids, polysaccharides and other substances that could interfere with common downstream applications.
Hard Facts .
- High quality, purity and yield of nucleic acids
- Quick and easy handling
- Automation possible
- Beads and kits for different applications

Applications .
With the MagSi-DNA beads and kits you can extract DNA from a wide variety of sample materials easily, quickly and in very high quality and purity.
For further details on the individual kits and areas of application please see below.
MagSi DNA beads and kits are also suited to extract RNA from a wide variety of sample materials easily, quickly and in very high quality and purity.
For further details on the individual kits and areas of application please see below.
Product overview Magnetic Beads for DNA Isolation .
Our kits for DNA purification .
| Product | DNA/RNA | Application | Sample material |
|---|---|---|---|
| MagSi-DNA Bead | DNA | Isolation of nucleic acids | Variabel |
| MagSi-NGSPREP Plus | DNA | Size selection and DNA clean-up in the NGS library preparation. | PCR reactions |
| MagSi-DT Removal | DNA | Dye terminator removal from BigDye®sequencing reactions. | Sequencing reactions |
| MagSi-DNA Body Fluid | DNA | Isolation of genomic DNA from blood, saliva or swab samples | Fresh or frozen as well as EDTA or citrate-stabilized whole blood, saliva, swab samples |
| MagSi-cfDNA | DNA | Isolation of cell-free circulating DNA from human plasma or serum samples | Human serum and plasma |
| MagSi-DX Pathogen / MagSi-NA Pathogen | DNA / RNA | Isolation of pathogenic DNA / RNA from various sample materials | Saliva, oral and nasopharyngeal swabs, serum and plasma |
| MagSi-DNA FFPE | DNA | Isolation of genomic DNA from FFPE-embedded mammalian tissue or cell samples | FFPE embedded tissue or cells |
| MagSi-DNA Plant CLS | DNA | Isolation of genomic DNA from plant samples | Plant seeds, leaf samples, root samples |
| MagSi-DNA Tissue & Cells | DNA | Isolation of genomic DNA from animal tissue samples and euraryotic cells | Animal tissue samples, eukaryotic cell material |
| MagSi-DNA Stool | DNA | Isolation of microbial DNA from stool samples | Stool |
| MagSi-DNA Animal | DNA | Isolation of genomic DNA from animal samples | Blood, semen, hair, saliva, swabs or lysed tissue |
Simple workflow, fast and cost-effective .
The purification of RNA and DNA using MagSi beads is very simple and fast.
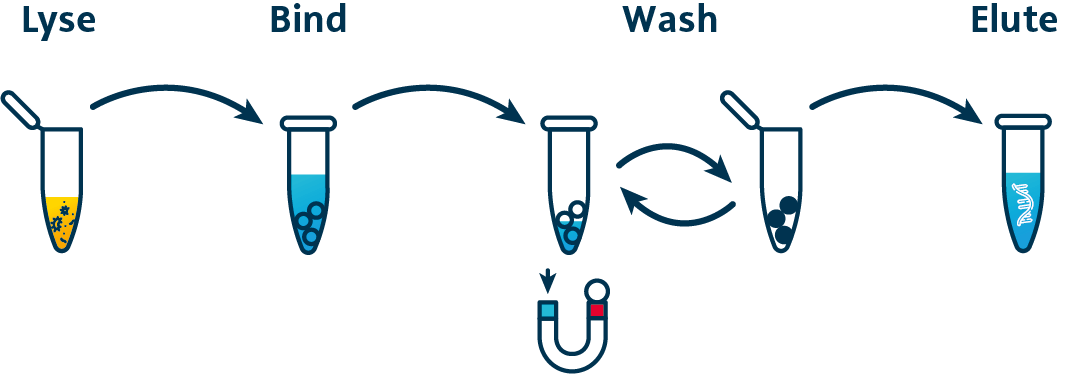
The protocol consists of only four steps: lysis, NA binding, washing, eluting.
The lysed sample material is mixed with the Magnetic Beads. The nucleic acids bind to the beads, which can be easily separated and washed using a magnet. In the final step, the bound DNA is released from the beads again using the elution buffer.
The MagSi-NA and the MagSi-DX Pathogen Kit can be used with Kingfisher™, PurePrep™, Hamilton® and TECAN® systems, among others.
MagSi DNA Beads .
In genetic analyses – especially in diagnostics or forensics – a high purity of DNA is necessary. Our MagSi-DNA beads meet this requirement excellently and reliably remove proteins, lipids, polysaccharides and other substances that possibly interfere with common downstream applications.
MagSi-DNA beads serve as a solid carrier phase in DNA extraction workflows. The beads are available with different physical properties and a silica or carboxyl modified surface.
- Quick and easy insulation/cleaning
- High quality and purity of DNA
- High yield
- Easily scalable
- Various beads and kits for special applications
MagSi-DNA Silica Beads for nucleic acid isolation from various sample materials (blood, cells, bacteria, etc.) can be used for manual and automated workflows.
Magnetic silica beads with increased surface area for longer suspension time.
| Item number | Product | Concentration | Bead size | Volume |
|---|---|---|---|---|
| MD01016 | MagSi-DNA 600 | 20 mg/mL | 600 nm | 2 mL |
| MD02016 | MagSi-DNA 600 | 20 mg/mL | 600 nm | 10 mL |
| MD03016 | MagSi-DNA 600 | 20 mg/mL | 600 nm | 100 mL |
Magnetic silica beads with fast separation and medium suspension time.
| Item numer | Product | Concentration | Bead size | Volume |
|---|---|---|---|---|
| MD01018 | MagSi-DNA allround | 20 mg/mL | 1.2 µm | 2 mL |
| MD02018 | MagSi-DNA allround | 20 mg/mL | 1.2 µm | 10 mL |
| MD03018 | MagSi-DNA allround | 20 mg/mL | 1.2 µm | 100 mL |
Magnetic silica beads with very fast separation and short suspension time.
| Item number | Product | Concentration | Bead size | Volume |
|---|---|---|---|---|
| MD01022 | MagSi-DNA 3.0 | 20 mg/ml | 3.0 µm | 2 ml |
| MD03022 | MagSi-DNA 3.0 | 20 mg/ml | 3.0 µm | 10 ml |
| MD04022 | MagSi-DNA 3.0 | 20 mg/ml | 3.0 µm | 100 ml |
Ferromagnetic silica beads are designed for use in microfluidic and chip-based genomic setups, but are also well suited for setups in tubes or microplates.
| Item number | Product | Bead size | Volume |
|---|---|---|---|
| MD0200010002 | MagSi-DNA mf | 300 nm | 2 ml |
| MD0200010010 | MagSi-DNA mf | 300 nm | 10 ml |
| MD0200010100 | MagSi-DNA mf | 300 nm | 100 ml |
MagSi DNA COOH Beads .
In genetic analyses – especially in diagnostics or forensics – a high purity of DNA is necessary. Our MagSi-DNA COOH magnetic beads meet this requirement perfectly, reliably removing proteins, lipids, polysaccharides and other substances that could interfere with common downstream applications.
- Quick and easy insulation/cleaning
- High quality and purity of DNA
- High yield
- Easily scalable
- Various beads and kits for special applications
Intended for nucleic acid isolation from various sample materials (blood, cells, bacteria, etc.) for manual and automated workflows. Under specific conditions, beads with carboxylated surfaces can lead to improved DNA/RNA yield and purity.
Magnetic COOH silica beads for a larger surface area and longer suspension time.
| Item number | Product | Concentration | Bead size | Volume |
|---|---|---|---|---|
| MD01021 | MagSi-DNA 600 COOH | 20 mg/ml | 600 nm | 2 ml |
| MD02021 | MagSi-DNA 600 COOH | 20 mg/ml | 600 nm | 10 ml |
| MD03021 | MagSi-DNA 600 COOH | 20 mg/ml | 600 nm | 100 ml |
Magnetic COOH-silica beads with fast separation and medium suspension time.
| Item numer | Product | Concentration | Bead size | Volume |
|---|---|---|---|---|
| MD01020 | MagSi-DNA allround COOH | 20 mg/ml | 1.2 µm | 2 ml |
| MD02020 | MagSi-DNA allround COOH | 20 mg/ml | 1.2 µm | 10 ml |
| MD03020 | MagSi-DNA allround COOH | 20 mg/ml | 1.2 µm | 100 ml |
Magnetic COOH-silica beads with very fast separation and short suspension time.
| Item number | Product | Concentration | Bead size | Volume |
|---|---|---|---|---|
| MD01024 | MagSi-DNA 3.0 COOH | 20 mg/ml | 3.0 µm | 2 ml |
| MD03024 | MagSi-DNA 3.0 COOH | 20 mg/ml | 3.0 µm | 10 ml |
| MD04024 | MagSi-DNA 3.0 COOH | 20 mg/ml | 3.0 µm | 100 ml |
Ferrimagnetic COOH silica beads are designed for use in microfluidic and chip-based genomic setups, but are also well suited for steups in tubes or microplates.
| Item numer | Product | Bead size | Volume |
|---|---|---|---|
| MD0200040002 | MagSi-DNA mf COOH | 300 nm | 2 ml |
| MD0200040010 | MagSi-DNA mf COOH | 300 nm | 10 ml |
| MD0200040100 | MagSi-DNA mf COOH | 300 nm | 100 ml |
MagSi-DNA Beads Trial Kit .
In genetic analyses – especially in diagnostics or forensics – a high purity of DNA is necessary. Our MagSi-DNA magnetic beads meet this requirement perfectly, reliably removing proteins, lipids, polysaccharides and other substances that could interfere with common downstream applications.
MagSi-DNA beads serve as a solid support phase in DNA extraction workflows consisting of the simple steps bind-wash-elute. The beads are available with different physical properties and a silica or carboxyl modified surface.
- Quick and easy insulation/cleaning
- High quality and purity of DNA
- High yield
- Easily scalable
- Various beads and kits for special applications
A complete set consisting of 8 different MagSi-DNA beads, offered in a single kit.
Suitable for the development of new extraction and purification protocols or as a replacement in existing protocols.
The kit contains the silica beads MagSi-DNA mf, MagSi-DNA 600, MagSi-DNA allround, MagSi-DNA 3.0 and the carboxylated beads MagSi-DNA mf COOH, MagSi-DNA 600 COOH, MagSi-DNA allround COOH, MagSi-DNA 3.0 COOH.
| Item number | Product | Bead size | Volume |
|---|---|---|---|
| MD06028 | MagSi-DNA Trial Kit | 300 nm, 600 nm, 1.2 µm and 3.0 µm | 8x 2 ml |
MagSi-NA / -DX Pathogens Kit .
Purification of DNA and RNA with the MagSi-DX or MagSi-NA Pathogens Kit provides nucleic acids with high purity and yield – ideal for qPCR-based or other enzymatic pathogen detection methods. All reagents are supplied ready-to-use and purification is based on a simple three-step protocol. The included MagSi-PA VII magnetic beads are optimized for rapid separation even from viscous sample lysates.
- Experimental time required for 96 samples: <30 min
- High yield of DNA and RNA for subsequent pathogen detection
- No cooling, centrifugation or filtration required
- Compatible with Kingfisher and other robots
- Works in lysates with high viscosity
- Suitable for many downstream applications
- CE/IVD certified (MagSi-DX Pathogens)
| Item number | Product | Amount |
|---|---|---|
| MDDX00010096 | MagSi-DX Pathogen | 96 preps |
| MDDX00010960 | MagSi-DX Pathogen | 10x 96 preps |
| MDDX0001005K | MagSi-DX Pathogen | 5000 preps |
| MDDX0001025K | MagSi-DX Pathogen | 25000 preps |
| MDKT00210096 | MagSi-NA Pathogens | 96 preps |
| MDKT00210960 | MagSi-NA Pathogens | 10x 96 preps |
| MDKT0021005K | MagSi-NA Pathogens | 5000 preps |
| MDKT0021025K | MagSi-NA Pathogens | 25000 preps |
| MDKT0021BULK | MagSi-NA Pathogens | Customized BULK |
MagSi DNA Vegetal Kit .
The MagSi-DNA Vegetal Kit enables the purification of nucleic acids from plant seeds and tissues such as leaves and roots. The included Lysis Buffer VG is optimized for the release of difficult to access nucleic acids from plant material. DNA and RNA extraction is performed by MagSi-VG III beads.
MagSi-DNA Vegetal Kits are optimized for the extraction of nucleic acids from seeds as well as plant material (e.g. leaves) of plants such as cucumber, bell pepper, tomato, wheat, flowers, sugar beet, canola, corn or potatoes. MagSi-DNA Vegetal II is better suited here for seed material, whereas MagSi-DNA Vegetal III has been optimized for plant material such as leaves and roots.
- Ready-to-use kit
- Optimized for seed or leaf material
- Efficient lysis due to specially developed buffer
- High yield
- High quality and purity of DNA
- Easy to automate and suitable for HTS
| Item number | Product | Amount | Sample |
|---|---|---|---|
| MDKT00060096 | MagSi-DNA Vegetal II | 96 preps | Seeds |
| MDKT00060960 | MagSi-DNA Vegetal II | 10x 96 preps | Seeds |
| MDKT00190096 | MagSi-DNA Vegetal III | 96 preps | Leaves, roots |
| MDKT00190960 | MagSi-DNA Vegetal III | 10x 96 preps | Leaves, roots |
MagSi DNA Body Fluid Kit .
The MagSi-DNA Body Fluid Kit enables fast and uncomplicated extraction of genomic DNA from blood, saliva or smear samples as well as cell culture and buffy coat. The ready-to-use kit shines with a simple and fast workflow and is suitable for manual and automated workflows.
- Ready-to-use kit
- Suitable for whole blood samples in citrate, heparin or EDTA tubes
- Suitable for saliva and other body fluids
- Fast and easy workflow - working time for 96 samples < 30 min
- High yield (up to 10 µg DNA from 200 µl blood), high quality and purity of DNA
- Suitable for HTS due to simple automation capability
- Compatible with liquid handling systems such as KingFisherTM, PrimaRWS®, PIPETMAX® and JANUS®.
| Item number | Product | Amount |
|---|---|---|
| MDKT00140096 | MagSi-DNA Body Fluids | 96 preps |
| MDKT00140960 | MagSi-DNA Body Fluids | 10x 96 preps |
| MDKT0014BULK | MagSi-DNA Body Fluids | BULK |
MagSi-DNA Tissue & Cells Kit .
The MagSi-DNA Tissue & Cells Kit allows easy purification of genomic DNA from eukaryotic cells and tissues without centrifugation or filtration. Processing time for preparation of 96 samples is approximately 40 minutes plus an additional 15 minutes of pre-lysis incubation (for cells) and 1-3 hours or overnight for tissue samples.
- Short and simple protocols, 96 samples in 40 minutes
- No phenol or chloroform extraction or alcohol precipitation required
- Eliminates the need for repeated centrifugation, vacuum or column separation
- Consistently high yield and purity of genomic DNA
- Excellent integrity DIN > 8.1
- Suitable for many downstream applications
- Easy to automate (PurePrep / KingFisherTM / Biosprint 96 / MagMaxTM protocols available)
- Compatible with general liquid handling robots (e.g. Hamilton®, TECAN®)
| Item number | Product | Amount |
|---|---|---|
| MDKT00180096 | MagSi-DNA Tissue & Cells | 96 preps |
| MDKT00180960 | MagSi-DNA Tissue & Cells | 10x 96 preps |
| MDKT0018BULK | MagSi-DNA Tissue & Cells | BULK – customized |
MagSi-cfDNA Kit .
The MagSi-cfDNA kit is intended for the (automated) purification of circulating cell-free DNA from human plasma or serum samples. The processing time for the preparation of 24 samples is about 60 minutes.
The kit does not require phenol/chloroform extraction or alcohol precipitation and eliminates the need for repeated centrifugation, vacuum filtration, or column separation. It allows safe handling of potentially infectious specimens. The cfDNA obtained can be used directly for downstream applications such as qPCR or any type of enzymatic reaction.
- Optimized for (automatic) use on PurePrep 24
- Suitable for use with fresh or frozen plasma or serum samples
- Plasma can be collected with different blood collection tubes (Streck Cell-Free DNA BCT, EDTA, Citrate etc.)
- Kit contains reagents for 96 extractions of cfDNA from 2 ml sample
- Scalable for use between 1 and 4 ml sample, support protocol available for 10 ml sample volume
- Typical yield: 0.5 to 4 ng cfDNA per ml human plasma (but varies greatly from donor to donor).
- After lysis at 56°C, all other steps are performed at RT
- Does not require carrier RNA
- Magnetic beads are supplied in an optimized storage buffer to reduce sedimentation time
- Suitable for many enzymatic downstream applications, especially RT-qPCR and sequencing.
- Processing time for 24 samples: ~60 min
- Kit is supplied complete (no additional alcohols required)
MagSi DNA FFPE Kit .
The MagSi-DNA FFPE Kit is optimized for manual and automated isolation of genomic DNA from mammalian FFPE tissue or cell samples. The processing time for 96 samples after kerosene removal and lysis is approximately 40 minutes.
The kit does not require phenol/chloroform extraction or alcohol precipitation and does not require centrifugation, vacuum filtration, or column separation either. The product is designed to avoid cross-contamination between samples to the greatest extent possible and to safely handle potentially infectious samples. The DNA obtained can be used directly in applications such as PCR, NGS or enzymatic reactions.
- Short and simple protocols, 96 samples in 40 minutes
- Safe handling of samples without the need for repeated centrifugation, vacuum filtration or column separation
- Consistently high yield and purity of genomic DNA
- Easy to automate
- Several options for dewaxing
- Depending on the sample material, RNA can be purified as well
- Protocols and consumables available for PurePrep/ KingFisherTM/ BioSprint 96/ MagMaxTM.
- Can be used on most common general liquid handling robots (e.g. Hamilton®, TECAN®)
| Item number | Product | Quantity |
|---|---|---|
| MDKT00240096 | MagSi-DNA FFPE | 96 preps |
| MDKT00240960 | MagSi-DNA FFPE | 10x 96 preps |
MagSi DNA Animal Kit .
MagSi-DNA Animal enables the rapid and cost-effective extraction of genomic DNA from various samples such as blood, semen, hair, saliva/swabs or lysed tissue. This universal DNA purification kit is optimized for extracting DNA from sample materials with the highest purity, providing DNA suitable for genotyping assays or other PCR-based analyses. The extraction chemistry has been validated on different species, e.g. horse, pig, dog, cattle, and can be adapted to any specific requirements in terms of yield, purity and working volume.
| Item number | Product | Amount |
|---|---|---|
| MDKT00150096 | MagSi-DNA Animal | 96 preps |
| MDKT00150960 | MagSi-DNA Animal | 10x 96 preps |
Magnetic DNA Beads and Kits .
DNA and RNA extraction for various applications.
Downloads .
Jones, J. C., Du, Z. G., Bernstein, R., Meyer, M., Hoppe, A., Schilling, E., Ableitner, M., Juling, K., Dick, R., Strauss, A. S., & Bienefeld, K. (2020). Tool for genomic selection and breeding to evolutionary adaptation: Development of a 100K single nucleotide polymorphism array for the honey bee. Ecology and Evolution, 10(13), 6246-6256. https://doi.org/10.1002/ece3.6357
Varberg, J. M., Gardner, J. M., McCroskey, S., Saravanan, S., Bradford, W. D., & Jaspersen, S. L. (2020). High-Throughput Identification of Nuclear Envelope Protein Interactions in Schizosaccharomyces pombe Using an Arrayed Membrane Yeast-Two Hybrid Library. G3 (Bethesda, Md.), 10(12), 4649-4663. https://doi.org/10.1534/g3.120.401880
Rombach, M., Hin, S., Specht, M., Johannsen, B., Lüddecke, J., Paust, N., Zengerle, R., Roux, L., Sutcliffe, T., Peham, J. R., Herz, C., Panning, M., Donoso Mantke, O., & Mitsakakis, K. (2020). RespiDisk: a point-of-care platform for fully automated detection of respiratory tract infection pathogens in clinical samples. The Analyst, 145(21), 7040-7047. https://doi.org/10.1039/d0an01226b
Ploi, K., Curto, M., Bolfíková, B. Č., Loudová, M., Hulva, P., Seiter, A., Fuhrmann, M., Winter, S., & Meimberg, H. (2020). Evaluating the Impact of Wildlife Shelter Management on the Genetic Diversity of Erinaceus europaeus and E. roumanicus in Their Contact Zone. Animals: An Open Access Journal from MDPI, 10(9), E1452. https://doi.org/10.3390/ani10091452
Jones, J. C., Du, Z. G., Bernstein, R., Meyer, M., Hoppe, A., Schilling, E., Ableitner, M., Juling, K., Dick, R., Strauss, A. S., & Bienefeld, K. (2020). Tool for genomic selection and breeding to evolutionary adaptation: Development of a 100K single nucleotide polymorphism array for the honey bee. Ecology and Evolution, 10(13), 6246-6256. https://doi.org/10.1002/ece3.6357
Jangam, S. (2018). Design of a Microfluidic Device for the Magnetic Extraction of DNA. https://doi.org/10.13140/RG.2.2.20345.31846
Tibihika, P. D., Curto, M., Dornstauder-Schrammel, E., Winter, S., Alemayehu, E., Waidbacher, H., & Meimberg, H. (2019). Application of microsatellite genotyping by sequencing (SSR-GBS) to measure genetic diversity of the East African Oreochromis niloticus. Conservation Genetics, 20(2), 357-372. https://doi.org/10.1007/s10592-018-1136-x
Curto, M., Winter, S., Seiter, A., Schmid, L., Scheicher, K., Barthel, L. M. F., Plass, J., & Meimberg, H. (2019). Application of a SSR-GBS marker system on investigation of European Hedgehog species and their hybrid zone dynamics. Ecology and Evolution, 9(5), 2814-2832. https://doi.org/10.1002/ece3.4960
Hui, T., Cao, Q., Wegrzyn-Woltosz, J., O’Neill, K., Hammond, C. A., Knapp, D. J. H. F., Laks, E., Moksa, M., Aparicio, S., Eaves, C. J., Karsan, A., & Hirst, M. (2018). High-Resolution Single-Cell DNA Methylation Measurements Reveal Epigenetically Distinct Hematopoietic Stem Cell Subpopulations. Stem Cell Reports, 11(2), 578-592. https://doi.org/10.1016/j.stemcr.2018.07.003
Hui, Z. K. (Tony). (2018). Epigenetic heterogeneity revealed through single-cell DNA methylation sequencing [University of British Columbia]. https://doi.org/10.14288/1.0365757
Engel, N. Y., Weiss, V. U., Wenz, C., Glück, S., Rüfer, A., Kratzmeier, M., Marchetti-Deschmann, M., & Allmaier, G. (2017). Microchip capillary gel electrophoresis combined with lectin affinity enrichment employing magnetic beads for glycoprotein analysis. Analytical and Bioanalytical Chemistry, 409(28), 6625-6634. https://doi.org/10.1007/s00216-017-0615-0
Kralj, S., Potrc, T., Kocbek, P., Marchesan, S., & Makovec, D. (2017). Design and Fabrication of Magnetically Responsive Nanocarriers for Drug Delivery. Current Medicinal Chemistry, 24(5), 454-469. https://doi.org/10.2174/0929867323666160813211736
Iranmanesh, I., Ohlin, M., Ramachandraiah, H., Ye, S., Russom, A., & Wiklund, M. (2016). Acoustic micro-vortexing of fluids, particles and cells in disposable microfluidic chips. Biomedical Microdevices, 18(4), 71. https://doi.org/10.1007/s10544-016-0097-4
>Mathot, L., Lindman, M., & Sjöblom, T. (2011). Efficient and scalable serial extraction of DNA and RNA from frozen tissue samples. Chemical Communications (Cambridge, England), 47(1), 547-549. https://doi.org/10.1039/c0cc02248a
Hui, T., Cao, Q., Wegrzyn-Woltosz, J., O’Neill, K., Hammond, C. A., Knapp, D. J. H. F., Laks, E., Moksa, M., Aparicio, S., Eaves, C. J., Karsan, A., & Hirst, M. (2018). High-Resolution Single-Cell DNA Methylation Measurements Reveal Epigenetically Distinct Hematopoietic Stem Cell Subpopulations. Stem Cell Reports, 11(2), 578-592. https://doi.org/10.1016/j.stemcr.2018.07.003
Jonckheere, I., Faes, L., Overmeire, Y., De Vleeschauwer, A., Vanden Daele, L., Van Bruaene, N., Vandecandelaere, I., Merlaen, B., van Cann, J., & Vandesompele, J. (2021). Equivalence of saliva RT-qPCR testing to nasal-throat/nasopharyngeal swab testing in the general practitioner’s setting to detect SARS-CoV-2 [Preprint]. Infectious Diseases (except HIV/AIDS). https://doi.org/10.1101/2021.09.30.21264181
Jones, J. C., Du, Z. G., Bernstein, R., Meyer, M., Hoppe, A., Schilling, E., Ableitner, M., Juling, K., Dick, R., Strauss, A. S., & Bienefeld, K. (2020). Tool for genomic selection and breeding to evolutionary adaptation: Development of a 100K single nucleotide polymorphism array for the honey bee. Ecology and Evolution, 10(13), 6246-6256. https://doi.org/10.1002/ece3.6357

